Direct Dyes
The direct dyes that are soluble in water and ionizable to form colored anions can directly dye the cellulose fibers or protein fibers without the action of the mordant.
This kind of dyes was developed after the find of the Congo red in 1884. The Congo red is formed by the couple of the united amine and 1-naphthylamine-4-sulfonic acid. To the 19th century, a variety of amino acid naphthol began the industrial production, and their varieties were greatly enriched. Green and black direct dyes had emerged.
The direct dyes have a higher affinity to the cellulose fibers, and the dyeing of the cellulose fibers should be carried out in a neutral or weakly alkaline medium. While the protein fibers are generally dyed in a neutral or weakly acidic medium.
The direct dyes are mostly sodium salts of aromatic compounds. They are soluble in water and their solubility increases with temperature. The direct dyes can dissociate into anions in water. The leather surface can be easily colored but it is generally difficult for the direct dyes to penetrate into the leather interior due to their big molecular weight and poor permeability. So we usually add a little ammonia before the dyeing to promote the penetration and slow down the combination with leather.
The direct dyes are sensitive to acid, and the addition of acid can make the direct dyes deposit. After dyeing, if we treat them with the fixing agent, organic acid, etc., we can improve the fastness.
The direct dyes are popular for their simple production method, complete chromatography, low cost and easy application, but their soaping fastness and light fastness are relatively poor. The direct dyes have been previously widely used for the dyeing of the cellulose fibers, protein fibers, synthetic fibers and their blended fabric. The main intermediates of many varieties of direct dyes are benzidine and its derivatives, which have been proposed as carcinogenic substances in the early 1970s and taken out of production for use. So far the production of direct dyes in the world's major companies have also been stopped and applied.
As for the different application, the direct dyes will be divided into four categories: general direct dyes, direct fast dyes, direct copper dyes and direct diazo dyes. According to the chemical structure, their types include azo, stilbene, phthalocyanine and dioxazine, of which the azo-type is the most important. In general, the main structure type of the direct dyes is azo (including monoazo and polyazo), and benzidine and its derivatives are the diazo component of many varieties. For example, Congo Red (C.I. Direct Red 28) ,the first direct dye synthesized by the N. Bottiger in 1884, has been no longer used for dyeing due to its poor light fastness, but it can be used for biological materials, colored paper as well as indicating acid agent.
The direct Fast Dyes refer to the light fastness of direct dyes in level 4 or more, and their molecular structure is more complex than the average direct dyes, such as the direct light Brilliant Blue (C.I. Direct Blue 106). The molecules of direct copper dyes contain groups that are capable of forming complex with the copper salt, such as hydroxyl group, carboxyl group or alkoxy group. They are in the mutual ortho-ortho-position of azo group. After copper treatment, the dyes can form copper complex and obtain better light fastness, such as the direct copper blue 2R (C.I. Direct Blue 151).
The molecules of fast direct diazo dyes contain an amino group. After dyeing on the fiber, the amino group may then couple with the coupled component on the fiber by diazotizationin to deepen the depth of the dye color and increases the dye fastness. Since the colors of this type of dyes show larger changes, it is difficult to control the color changes, and now they have been basically no longer used, such as direct diazo black BH (C. I. Direct Blue 2).
Click on the specific product, view the latest prices of the products, information, serving information
The direct dyes that are soluble in water and ionizable to form colored anions can directly dye the cellulose fibers or protein fibers without the action of the mordant.
This kind of dyes was developed after the find of the Congo red in 1884. The Congo red is formed by the couple of the united amine and 1-naphthylamine-4-sulfonic acid. To the 19th century, a variety of amino acid naphthol began the industrial production, and their varieties were greatly enriched. Green and black direct dyes had emerged.
The direct dyes have a higher affinity to the cellulose fibers, and the dyeing of the cellulose fibers should be carried out in a neutral or weakly alkaline medium. While the protein fibers are generally dyed in a neutral or weakly acidic medium.
The direct dyes are mostly sodium salts of aromatic compounds. They are soluble in water and their solubility increases with temperature. The direct dyes can dissociate into anions in water. The leather surface can be easily colored but it is generally difficult for the direct dyes to penetrate into the leather interior due to their big molecular weight and poor permeability. So we usually add a little ammonia before the dyeing to promote the penetration and slow down the combination with leather.
The direct dyes are sensitive to acid, and the addition of acid can make the direct dyes deposit. After dyeing, if we treat them with the fixing agent, organic acid, etc., we can improve the fastness.
The direct dyes are popular for their simple production method, complete chromatography, low cost and easy application, but their soaping fastness and light fastness are relatively poor. The direct dyes have been previously widely used for the dyeing of the cellulose fibers, protein fibers, synthetic fibers and their blended fabric. The main intermediates of many varieties of direct dyes are benzidine and its derivatives, which have been proposed as carcinogenic substances in the early 1970s and taken out of production for use. So far the production of direct dyes in the world's major companies have also been stopped and applied.
As for the different application, the direct dyes will be divided into four categories: general direct dyes, direct fast dyes, direct copper dyes and direct diazo dyes. According to the chemical structure, their types include azo, stilbene, phthalocyanine and dioxazine, of which the azo-type is the most important. In general, the main structure type of the direct dyes is azo (including monoazo and polyazo), and benzidine and its derivatives are the diazo component of many varieties. For example, Congo Red (C.I. Direct Red 28) ,the first direct dye synthesized by the N. Bottiger in 1884, has been no longer used for dyeing due to its poor light fastness, but it can be used for biological materials, colored paper as well as indicating acid agent.
The direct Fast Dyes refer to the light fastness of direct dyes in level 4 or more, and their molecular structure is more complex than the average direct dyes, such as the direct light Brilliant Blue (C.I. Direct Blue 106). The molecules of direct copper dyes contain groups that are capable of forming complex with the copper salt, such as hydroxyl group, carboxyl group or alkoxy group. They are in the mutual ortho-ortho-position of azo group. After copper treatment, the dyes can form copper complex and obtain better light fastness, such as the direct copper blue 2R (C.I. Direct Blue 151).
The molecules of fast direct diazo dyes contain an amino group. After dyeing on the fiber, the amino group may then couple with the coupled component on the fiber by diazotizationin to deepen the depth of the dye color and increases the dye fastness. Since the colors of this type of dyes show larger changes, it is difficult to control the color changes, and now they have been basically no longer used, such as direct diazo black BH (C. I. Direct Blue 2).
Click on the specific product, view the latest prices of the products, information, serving information
| STRUCTURE | CHEMICAL NAME | CAS | MF |
|---|---|---|---|
| Direct Grey GL | |||
| Direct Brilliant Yellow 2G | |||
| Direct Fast Blue | |||
| DIRECT BLACK 170 | |||
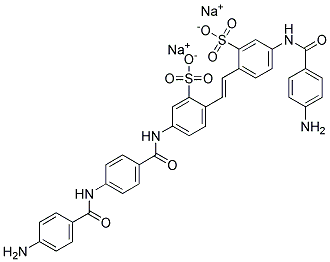 | DIRECT YELLOW 62 (C.I. 36900) | 6409-90-1 | C35H27N5Na2O9S2 |
| Direct Blue 199 | 12222-04-7 | ||
| Direct Blue 219 | |||
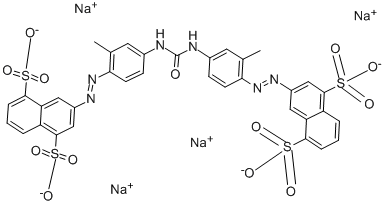 | DIRECT YELLOW 50 | 3214-47-9 | C35H24N6Na4O13S4 |
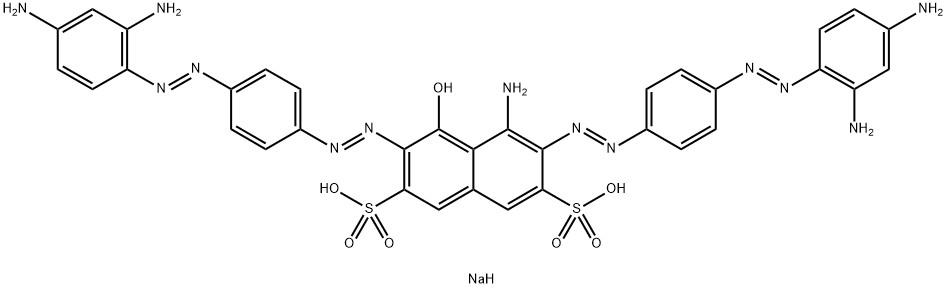 | Direct Black 19 | 6428-31-5 | C34H27N13Na2O7S2 |
 | CALCOMINE DARK GREEN BG | C34H23N7Na2O8S2 | |
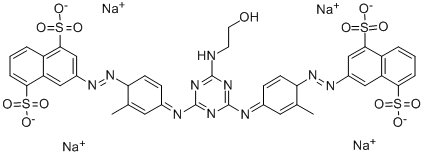 | Direct Yellow 86 | 50925-42-3 | C39H30N10Na4O13S4 |
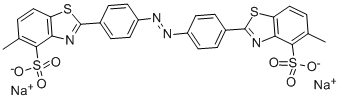 | Direct Yellow 28 | 8005-72-9 | C28H18N4O6S4.2Na |
 | Direct Green 59 | 7219-11-6 | C47H31N12O17S3.5Na |
| Direct Tangerine | |||
| Direct Black ET | |||
| amanil developed black bhsw | 6426-71-7 | ||
| DIRECT BLACK DYES | |||
| Direct Pink 5B | C32H20O8N5S2Na2 | ||
| DIRECT FAST BLACK G | C34H27O7N13S2Na2 | ||
| Direct Chestnut Brown | |||
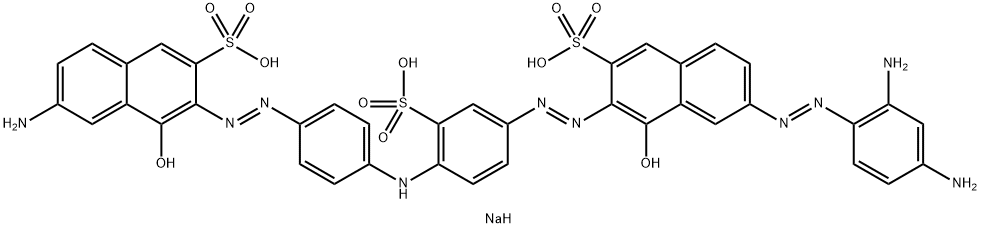 | Direct Black M,Tardirect Black M | 6897-38-7 | C38H27N10Na3O11S3 |
| Direct Blending Series |






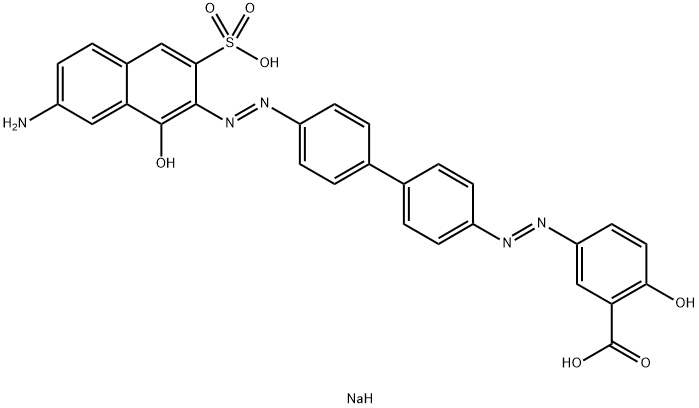
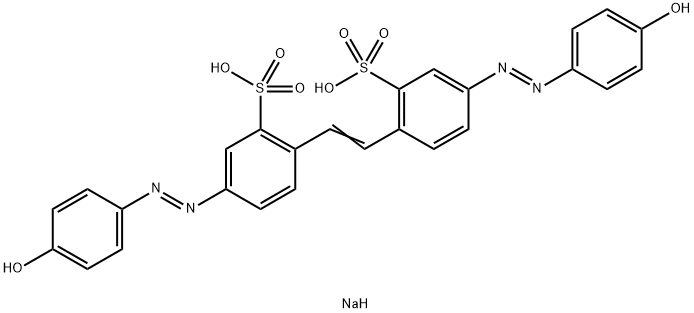
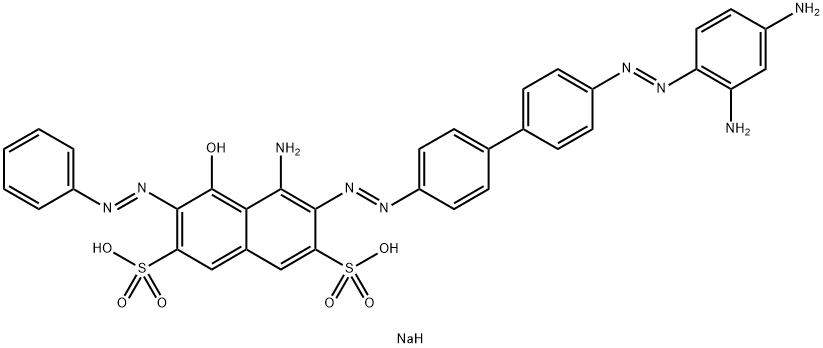
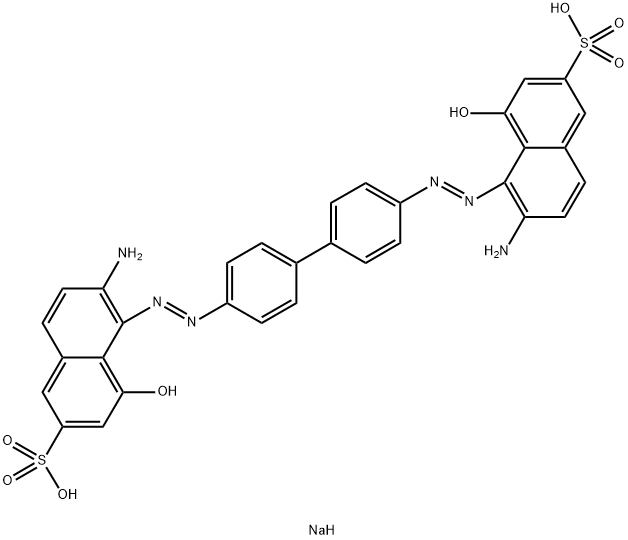
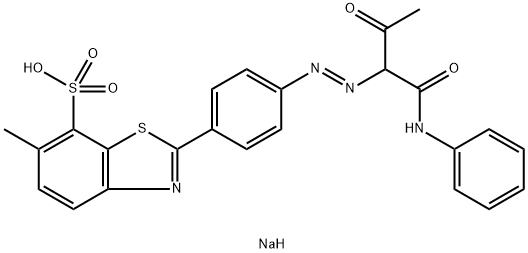
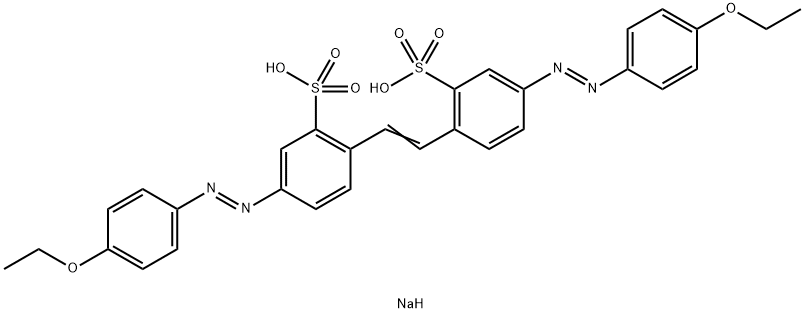
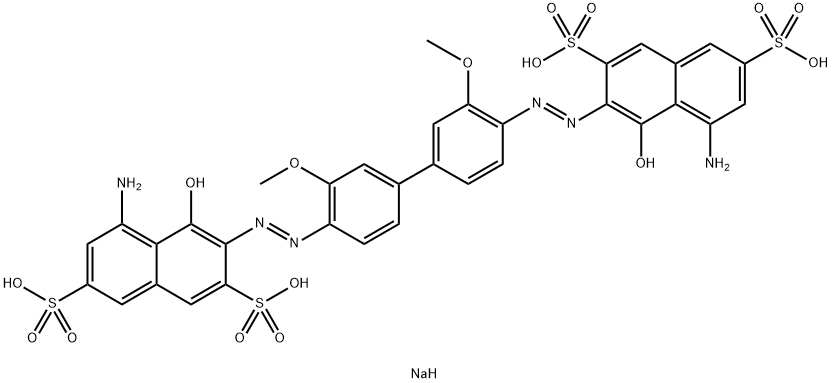
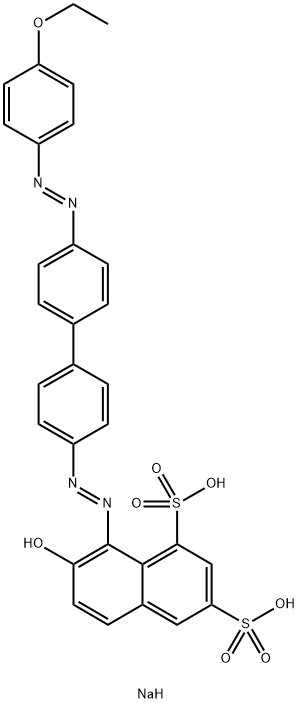

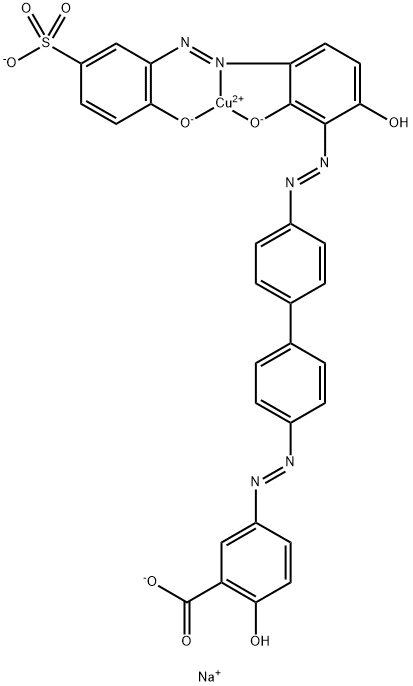
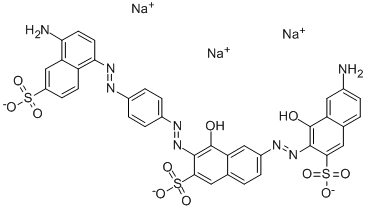
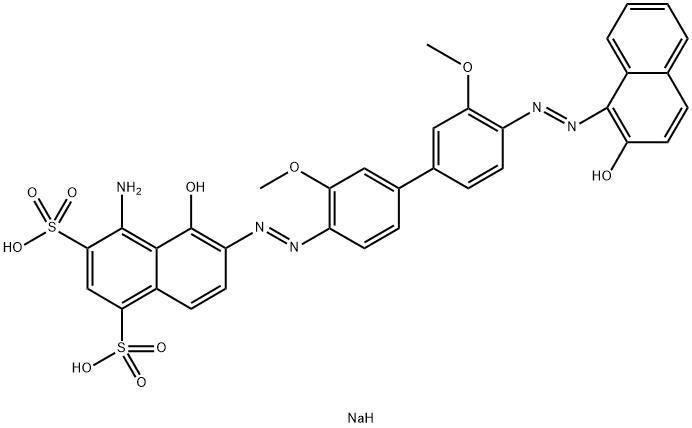
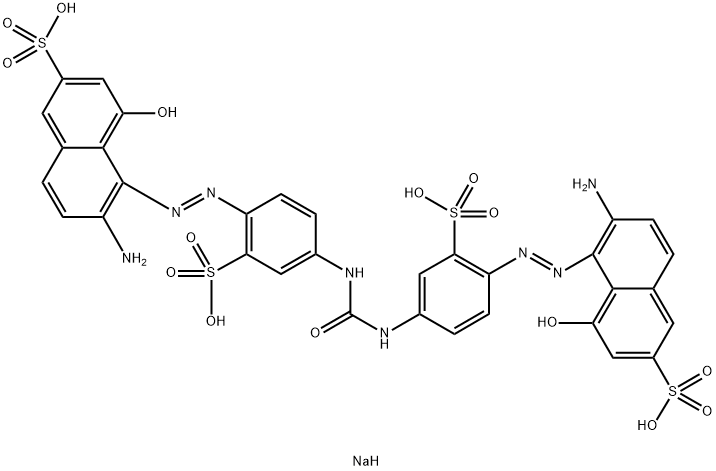
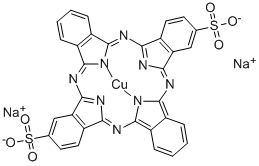
![tetrasodium 3,3'-[(1,6-dihydro-6-oxo-1,3,5-triazine-2,4-diyl)bis[imino(5-methoxy-2-methyl-4,1-phenylene)azo]]bis(naphthalene-1,5-disulphonate)](https://www.chemicalbook.com/CAS/GIF/82944-42-1.gif)
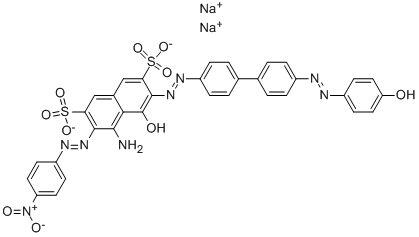
![disodium 2-[4-[[1-[[(2-methoxy-5-methyl-4-sulphonatophenyl)amino]carbonyl]-2-oxopropyl]azo]phenyl]-6-methylbenzothiazole-7-sulphonate](https://www.chemicalbook.com/CAS/GIF/72705-26-1.gif)
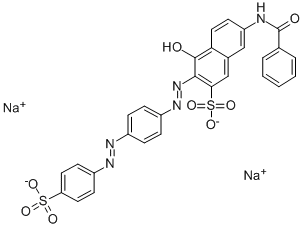
![disodium 4-amino-5-hydroxy-3-[[4'-[(4-hydroxyphenyl)azo][1,1'-biphenyl]-4-yl]azo]-6-(phenylazo)naphthalene-2,7-disulphonate](https://www.chemicalbook.com/CAS/GIF/3626-28-6.gif)
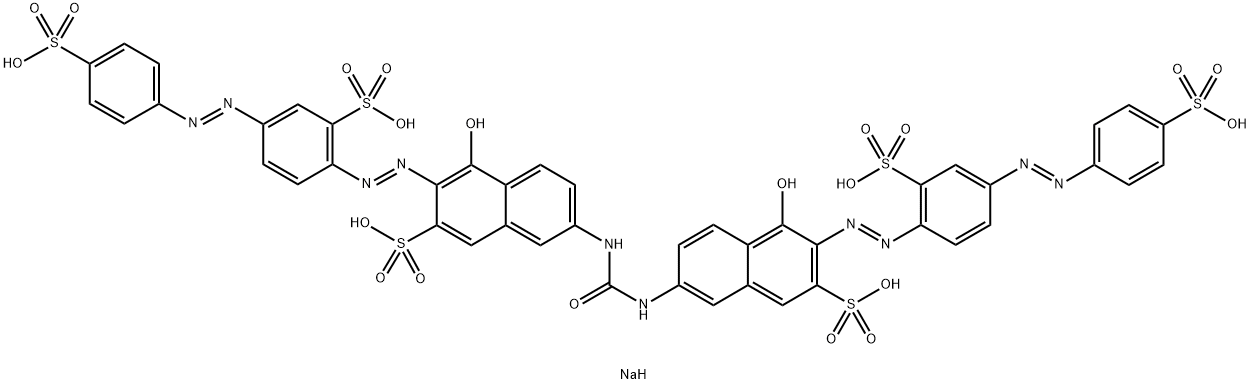
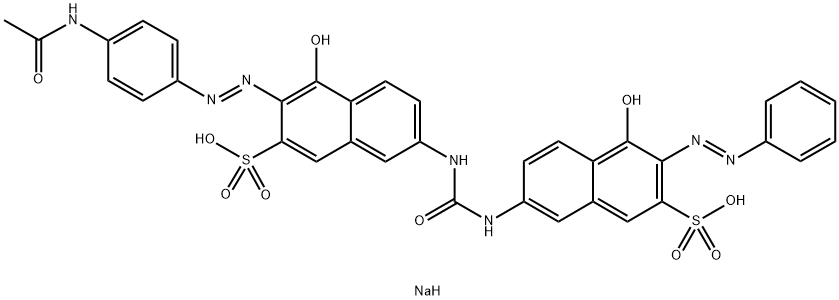
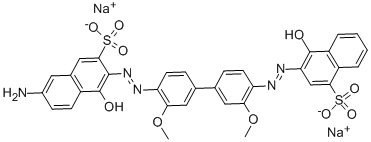
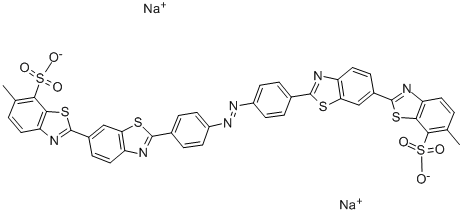
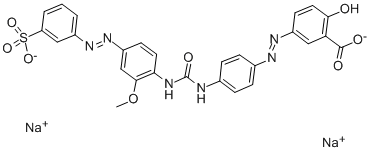
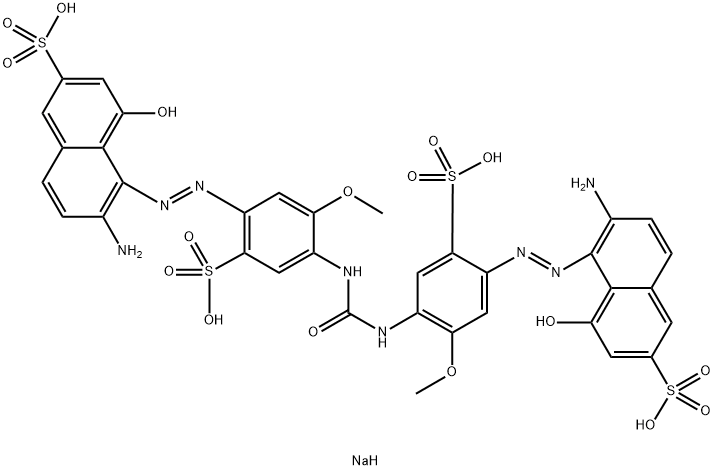
![disodium 3-[[4-[[4-amino-6(or 7)-sulphonatonaphthyl]azo]phenyl]azo]-6-[(2,4-diaminophenyl)azo]-4-hydroxynaphthalene-2-sulphonate](https://www.chemicalbook.com/CAS/20180808/GIF/8003-62-1.gif)
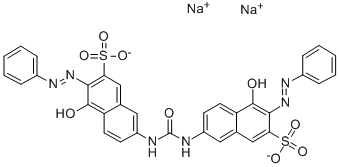
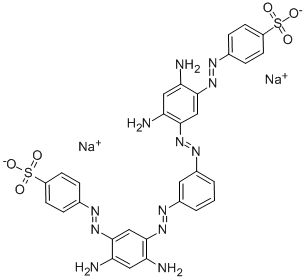
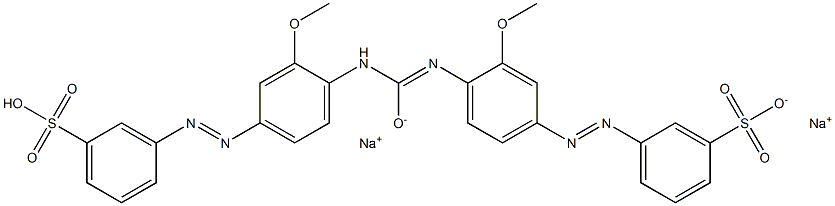

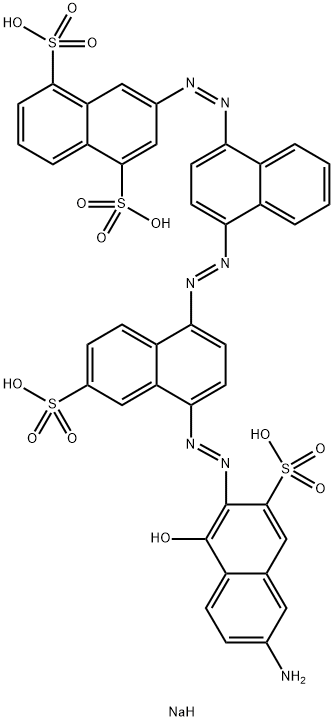
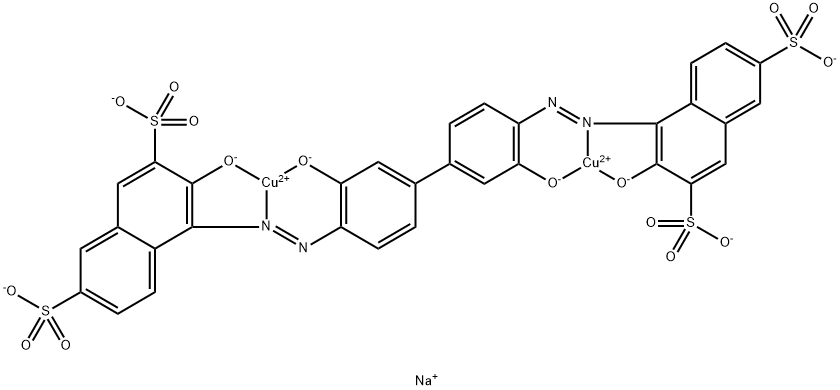
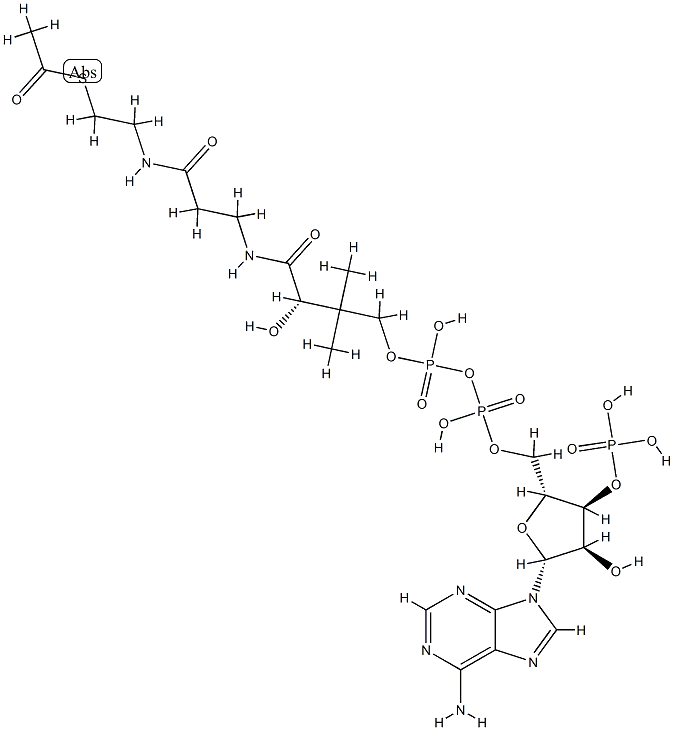
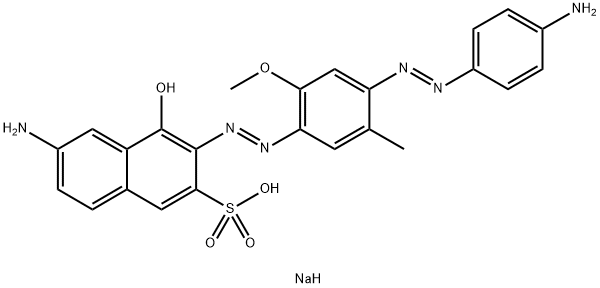
![tetrasodium 2-[[4-[[4-[[1-hydroxy-6-(phenylamino)-3-sulphonato-2-naphthyl]azo]-7-sulphonato-1-naphthyl]azo]-1-naphthyl]azo]benzene-1,4-disulphonate](https://www.chemicalbook.com/CAS/GIF/6428-58-6.gif)
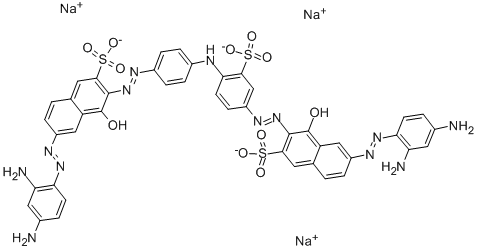
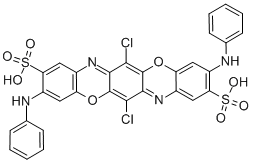
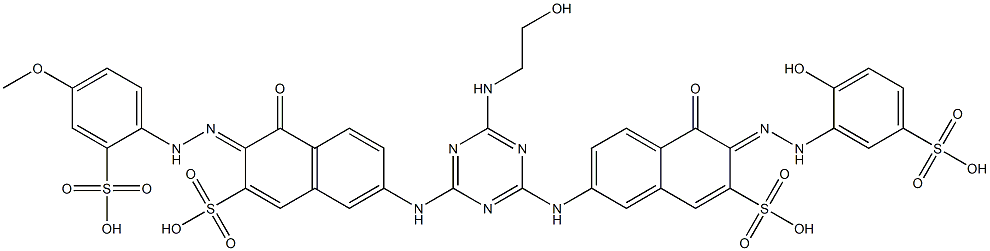
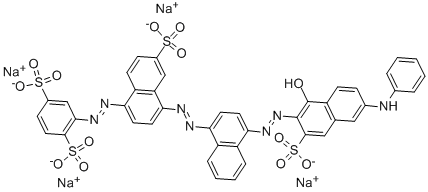
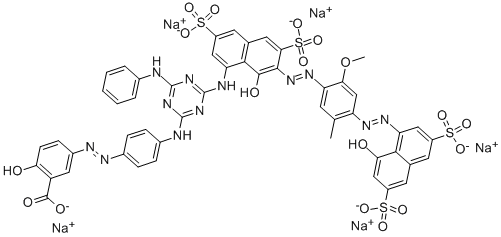
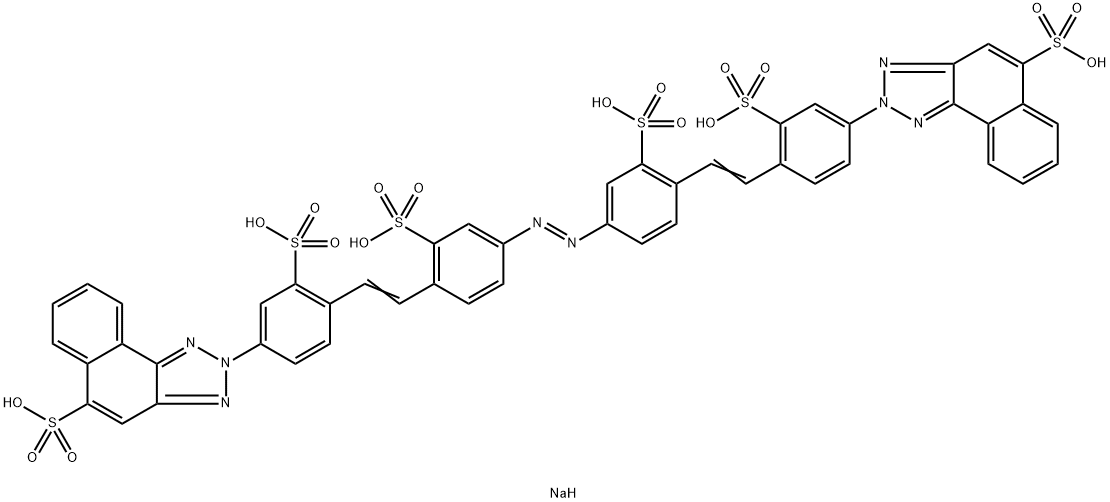
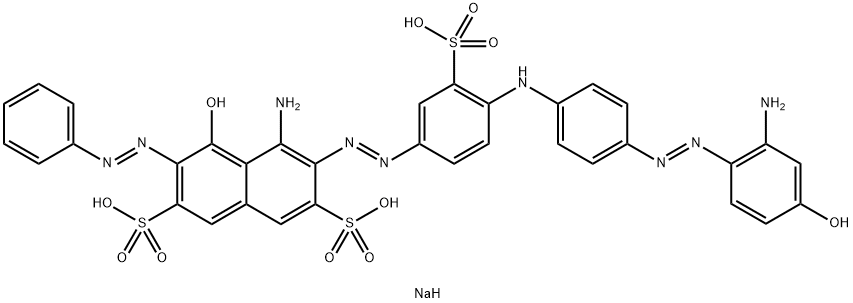
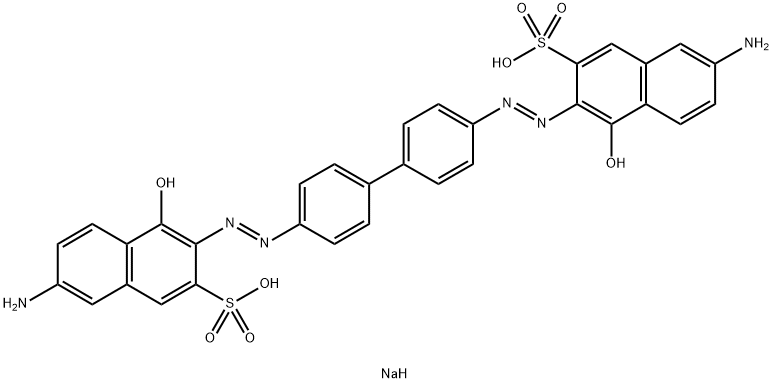
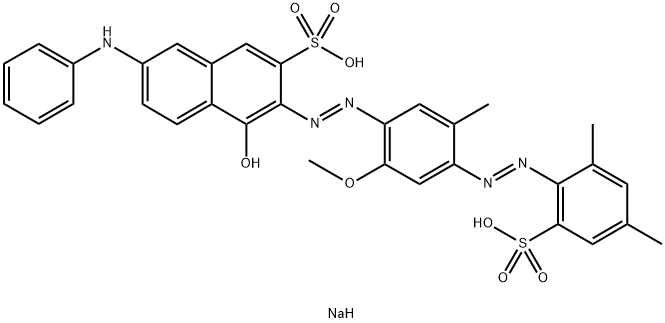
![tetrasodium 4,4'-[carbonylbis[imino(5-methoxy-2-methyl-4,1-phenylene)azo]]bis[5-hydroxynaphthalene-2,7-disulphonate]](https://www.chemicalbook.com/CAS/GIF/1937-34-4.gif)
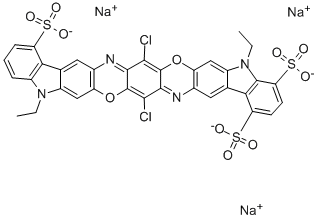
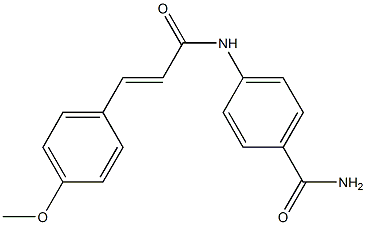
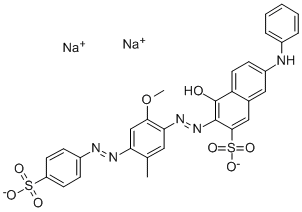

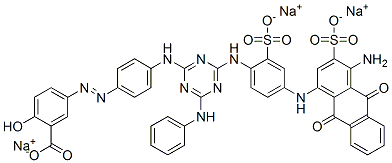

![tetrasodium [mu-[[7,7'-(carbonyldiimino)bis[4-hydroxy-3-[(2-hydroxy-5-sulphophenyl)azo]naphthalene-2-sulphonato]](8-)]]dicuprate(4-)](https://www.chemicalbook.com/CAS/GIF/15418-16-3.gif)

